Biosortia’s BSP1-003ND1s lead is a non-peptidic (undisclosed scaffold) small molecule that reproducibly returns a polypharmacology signature across independent in-silico screens: MC4R/MC3R (±MC5R) rank at the top (satiety/ energy-expenditure), GHSR appears frequently (hunger signaling), and OPRM1/OPRD1 are repeatedly returned (hedonic drive/reward). 5-HT2C (HTR2C) and C3AR1 surface in subsets of analogs as supportive nodes. While in-silico outputs are probability-based (not evidence of efficacy), this convergence supports a working MoA of MC4/3 agonism + GHSR antagonism + opioid-axis modulation (with NPY1R antagonism to be confirmed). The scaffold shows oralleaning (undisclosed) properties (cLogP/TPSA/rotors compatible with oral exposure), and early in-silico safety flags (e.g., EDNRA/MMPs in a few analogs) guide a focused counter-screening plan. Net-net, this is a credible, potential first-in-class oral polypharmacology program for weight loss, with controlled-release (CR) dosing as an option to smooth satiety signals and improve adherence.
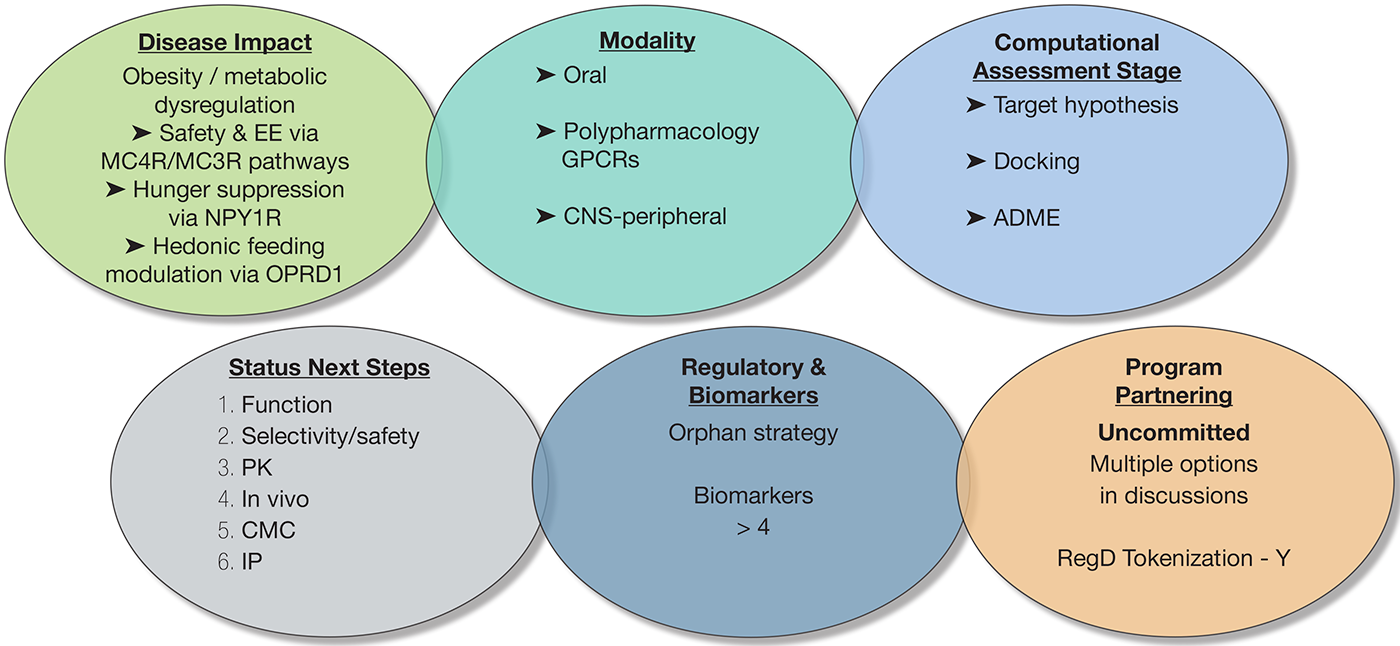
Next Steps:
- Mechanism & Potency : Quantify MC4R/MC3R cAMP EC50 (± bias), confirm NPY1R antagonism and OPRD1 modulation with orthogonal assays.
- Selectivity & Safety : Run broad off-target panel (e.g., CEREP/Eurofins), hERG, Ames, CYP/TDI, microsome/hepatocyte stability; human/rat microsomes and CYP/TDI.
- PK & Formulation : Establish oral exposure (F%, t1⁄2, dose linearity) in rodent (± dog); initiate salt/polymorph screen and early XR concept.
- In Vivo Signal : 2–4-week DIO mouse (food intake, body weight, indirect calorimetry, glucose/insulin, tolerability readouts).
- Chemistry & CMC : Lock a ≤8-step route, deliver >10 g non-GMP, stress/forced-degradation map, reference standards.
- IP & Regulatory : File CoM + degradants/analogs + combo claims; prepare ODD package for MC4R-axis obesity and schedule early agency interactions; organize a partner-ready data brief for option-to-license/co-dev.

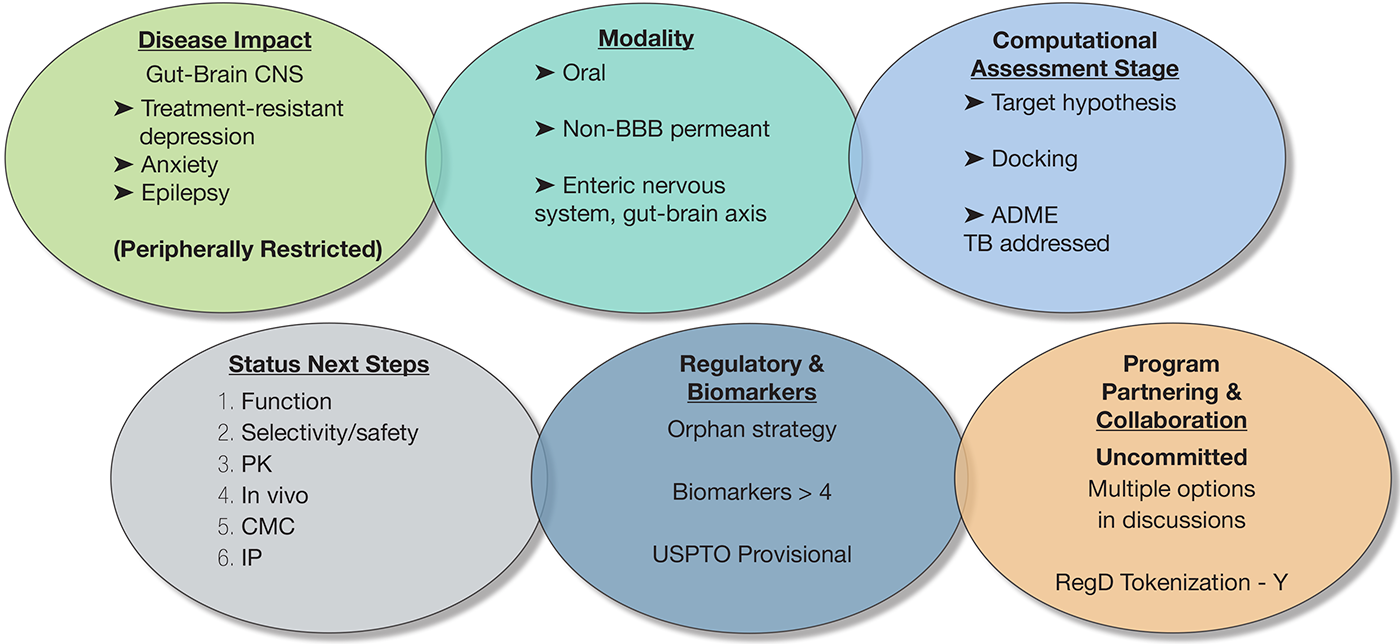
Next Steps:
The program is currently at the preclinical discovery stage, with all evidence based on computational modeling. The immediate next steps are focused on achieving in vitro proof-of-concept through a series of critical, de-risking experiments:
- Function : Experimentally confirm the predicted multi-activity profile through cell-based binding and functional assays
- Safety & Selectivity : Validate the selectivity and de-risk early safety liabilities via broad-panel off-target screening and preliminary in vitro cardiac safety (hERG) assessment.
- Pharmacokinetics (PK) : Prove the core therapeutic hypothesis by experimentally confirming the molecule is not blood-brain barrier permeant using an in vitro model (e.g., PAMPA-BBB).
- CMC & IP : Enable all testing by executing the first multi-gram synthesis and use the resulting experimental data to strengthen the program’s intellectual property position.

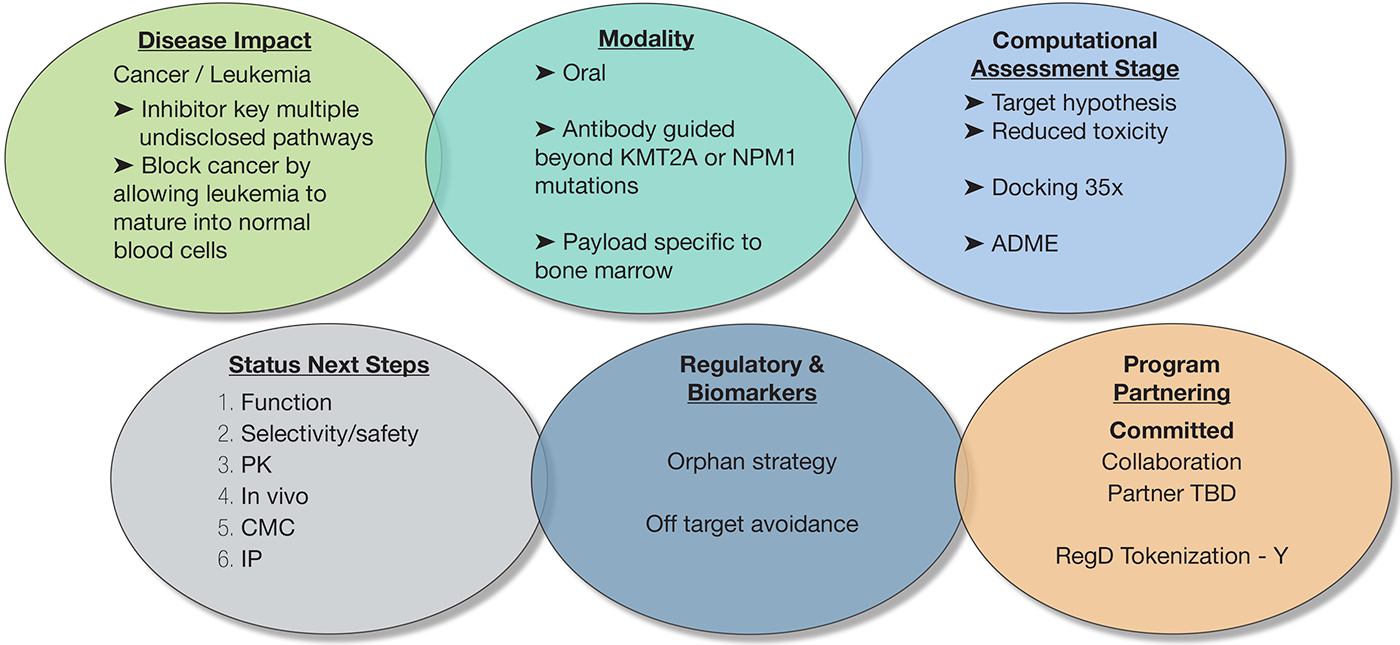
Next Steps:
- Validate On-Target ADC Activity: Confirm successful antibody targeting, internalization, and intracellular payload release in relevant leukemia cell lines.
- Demonstrate In Vivo Proof-of-Concept: Establish superior anti-leukemia efficacy and survival benefit in patient-derived xenograft (PDX) animal models.
- Define Therapeutic Window: Conduct initial in vivo toxicology and pharmacokinetic (PK) studies to establish a preliminary safety margin and dosing strategy.
- Confirm Target Patient Population: Verify ADC effectiveness across a panel of diverse leukemia subtypes to define the intended clinical population beyond known mutations.
- Develop a Scalable Manufacturing Process: Secure a consistent and reproducible Chemistry, Manufacturing, and Controls (CMC) process for the antibody, payload, and final ADC.
- Align with Regulatory Agencies: Initiate a pre-IND (Investigational New Drug) meeting with the FDA to get crucial feedback on the preclinical data package and clinical trial design.


